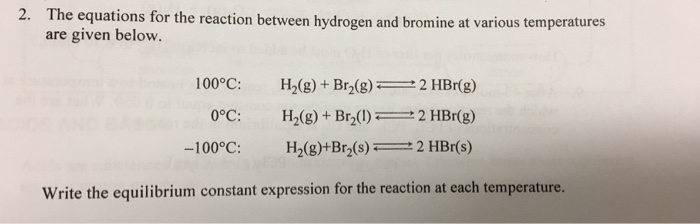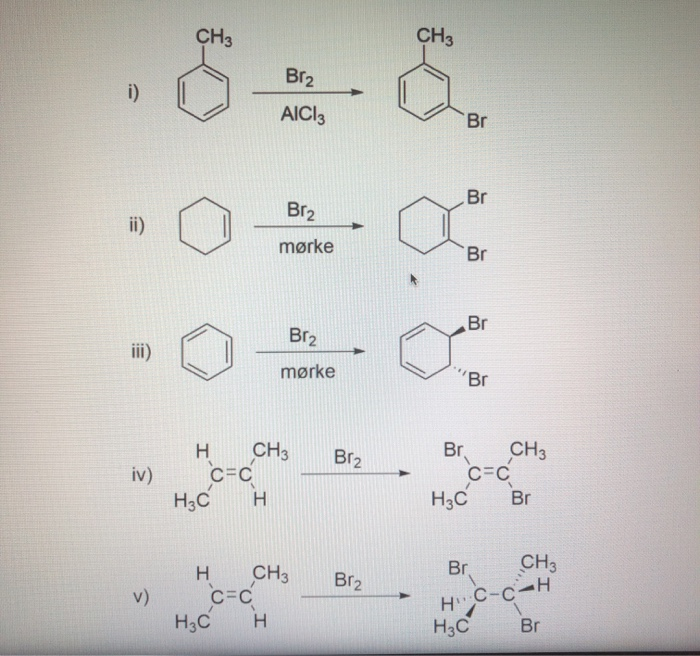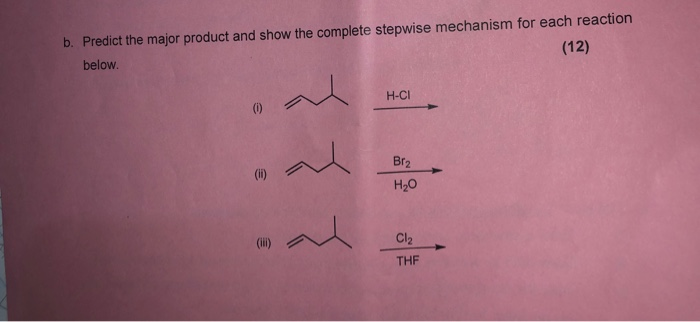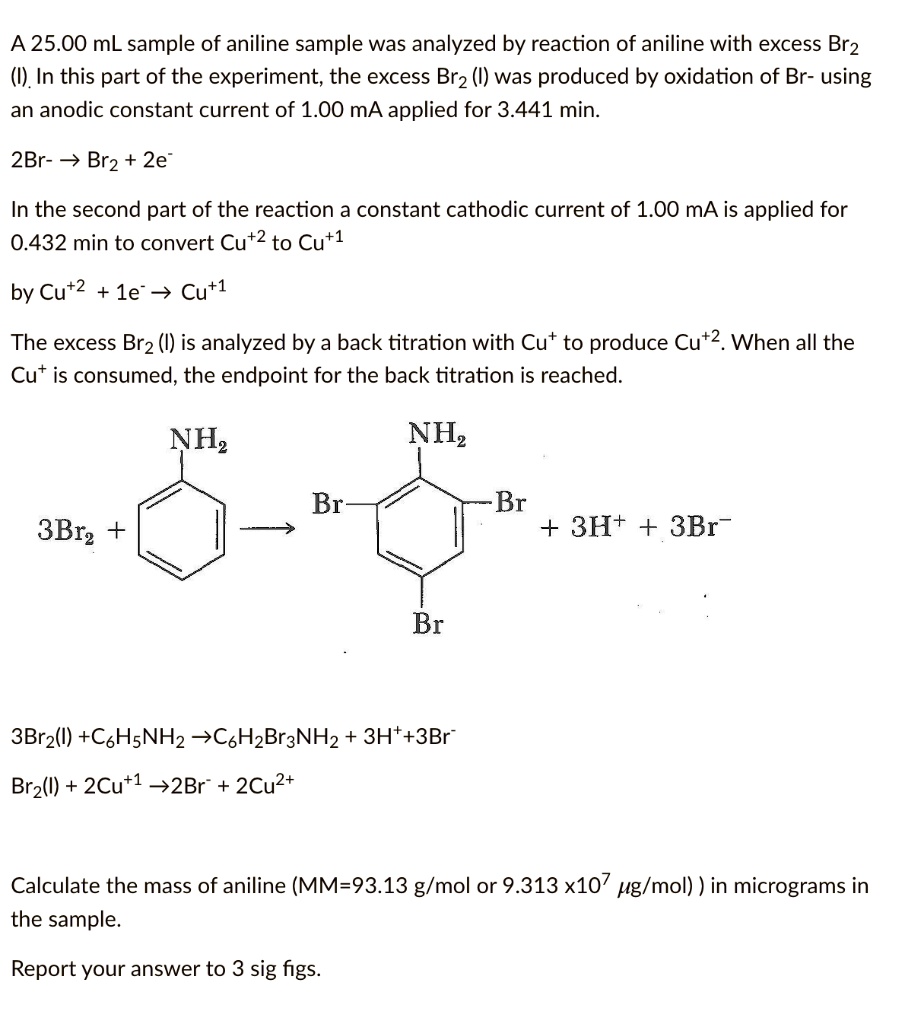
SOLVED: A 25.00 mL sample of aniline sample was analyzed by reaction of aniline with excess Br2 (I). In this part of the experiment; the excess Brz (I) was produced by oxidation
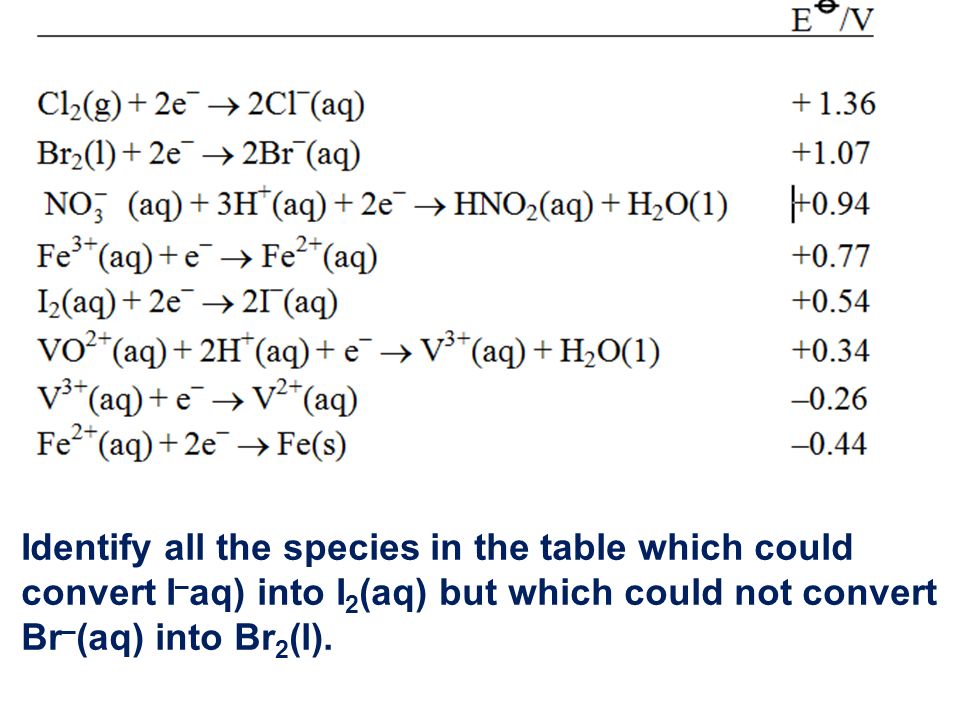
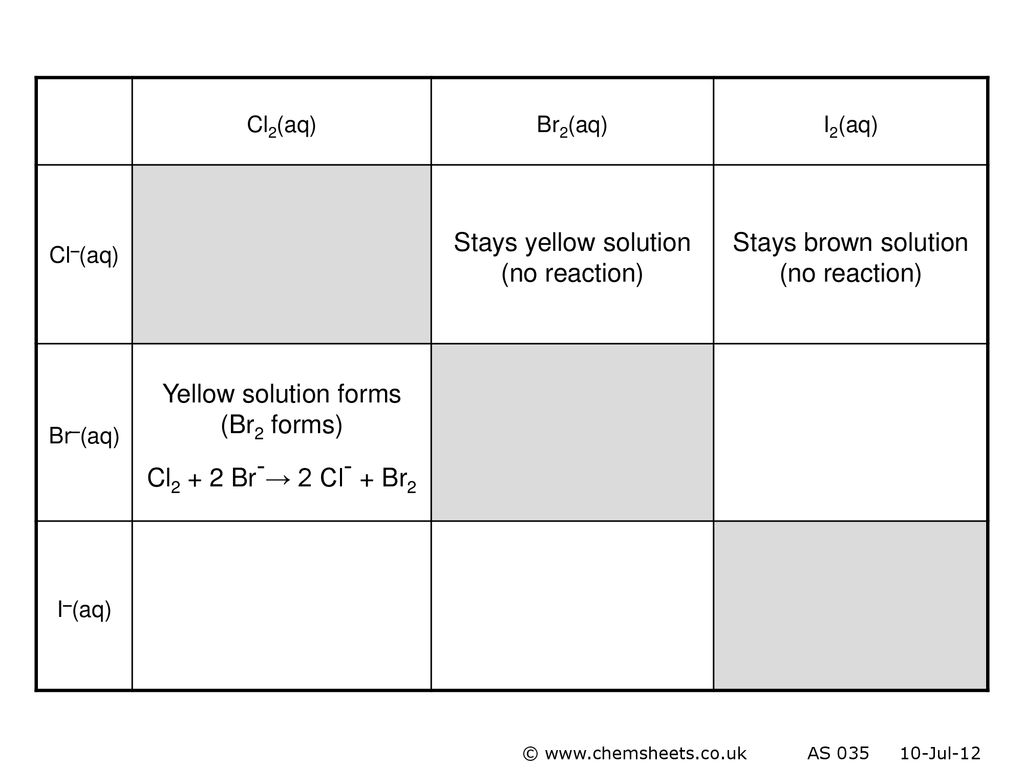
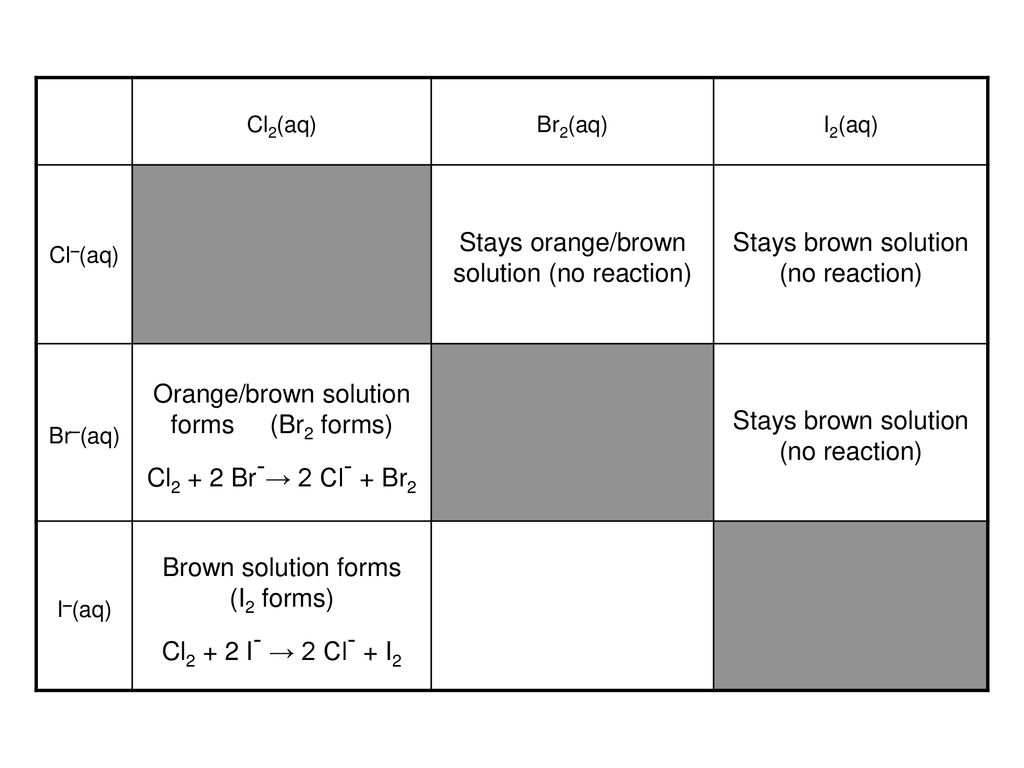
Q5 from 5.3 Identify all the species in the table which could convert I–aq) into I2(aq) but which could not convert Br–(aq) into Br2(l). - ppt video online download

⚗️Which of these statements are true? Select all that apply. The Delta.Hf for Br2(I) is 0 kJ/mol. The - Brainly.com

Bromine Radical (Br• and Br2•–) Reactivity with Dissolved Organic Matter and Brominated Organic Byproduct Formation | Environmental Science & Technology

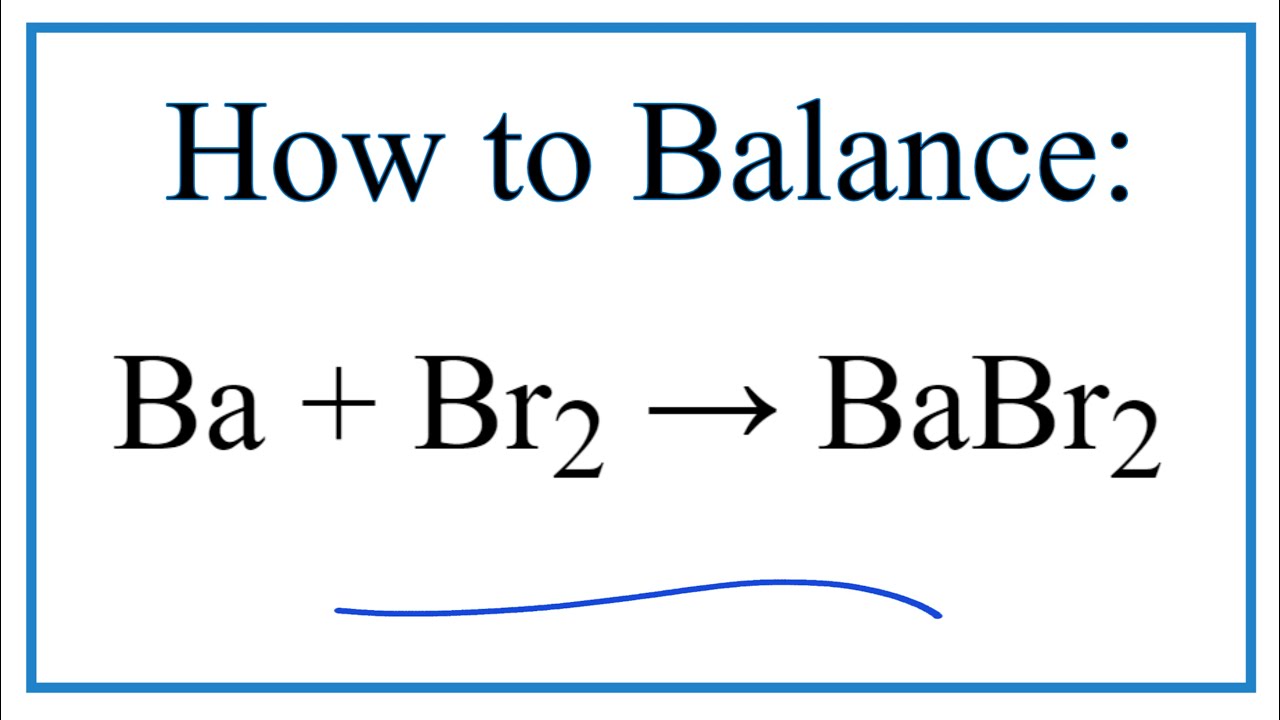
SOLVED: determine the substance reduced this redox reaction 2 NaBr (s) + Cl2 (g) —> Br2 (I) + 2 NaCI (s) NaBr, Na, Cl2, NaCl, Br