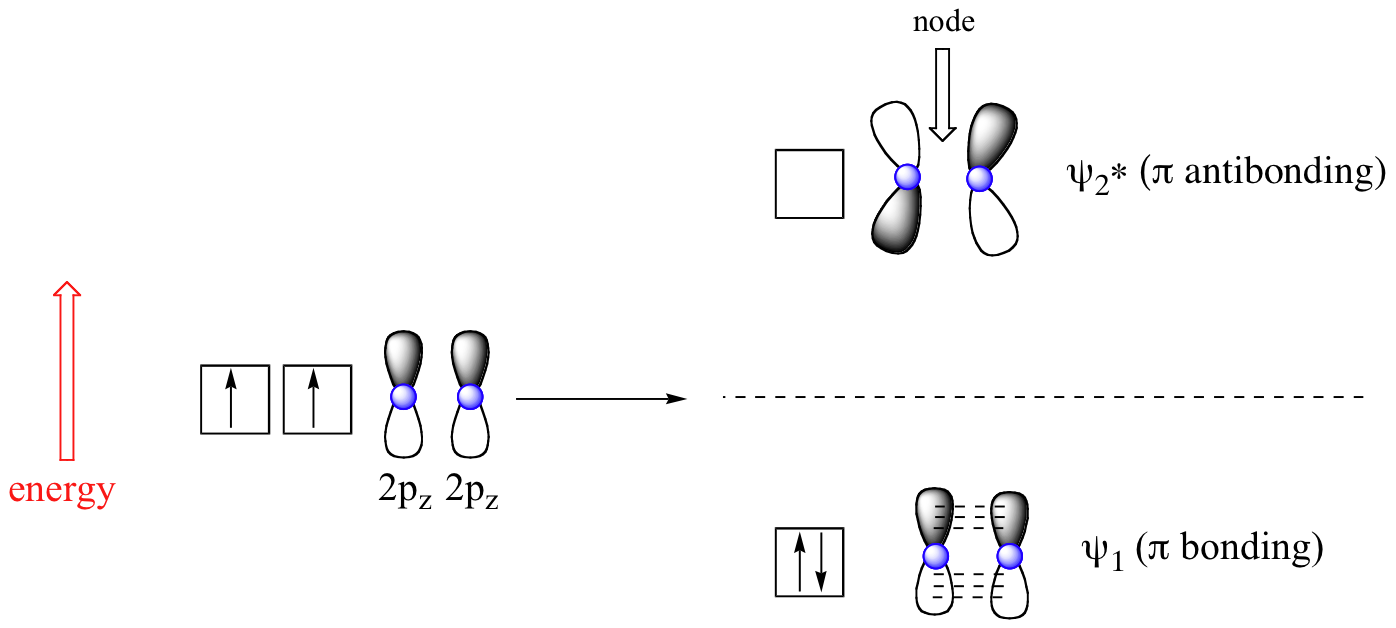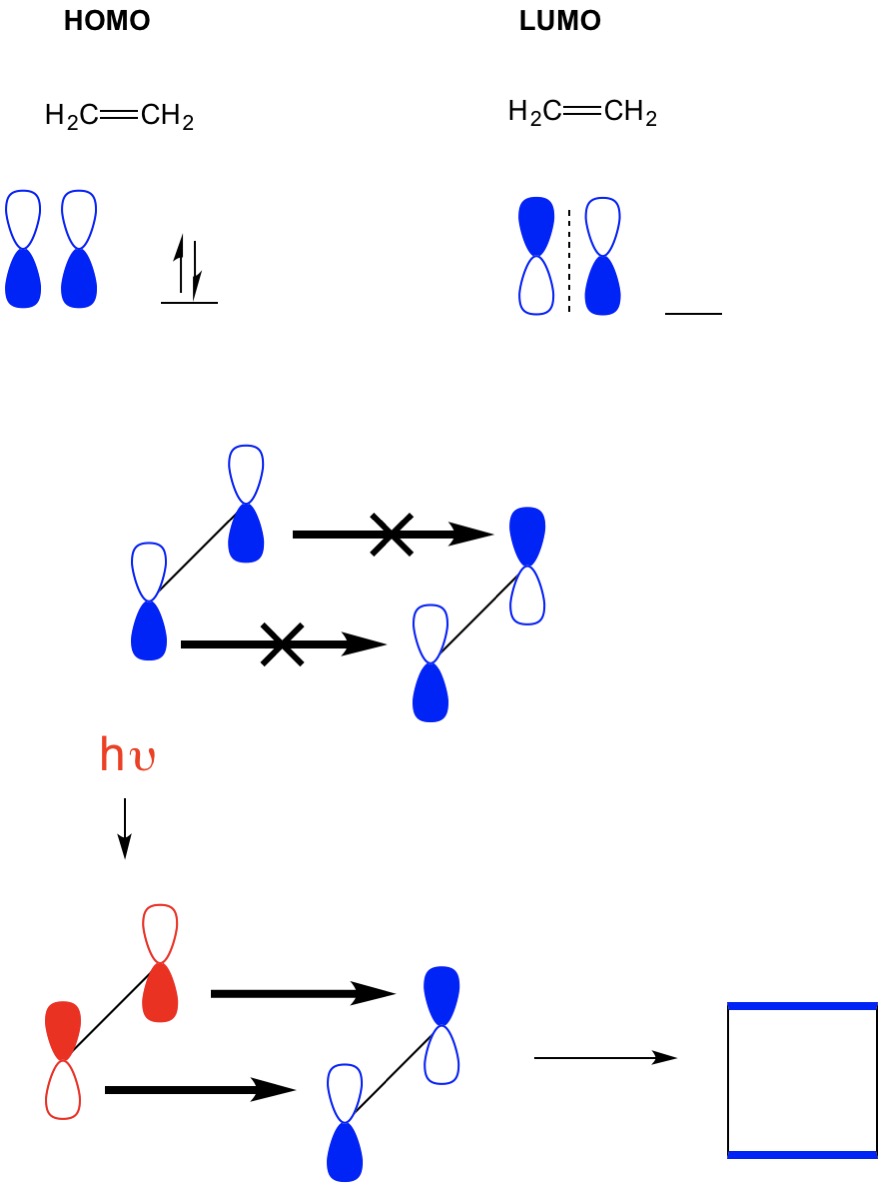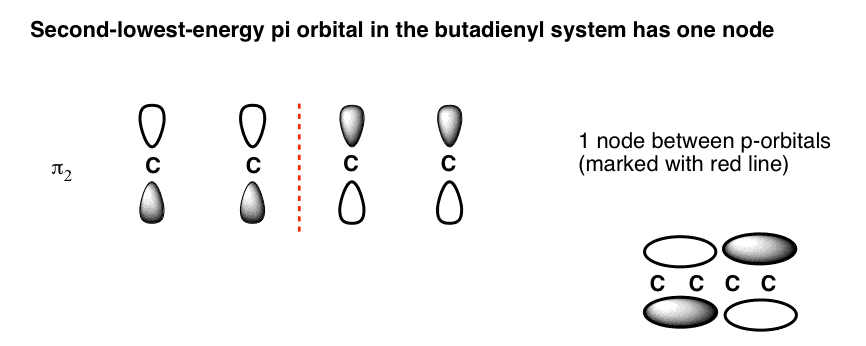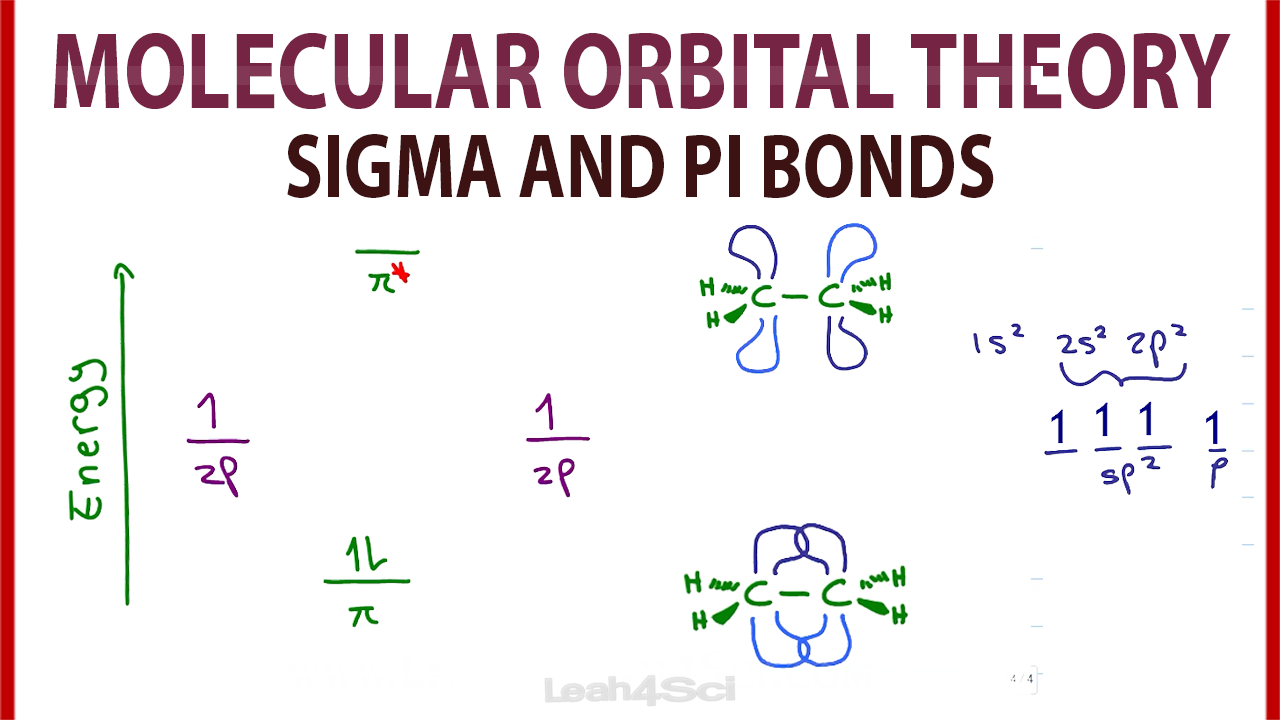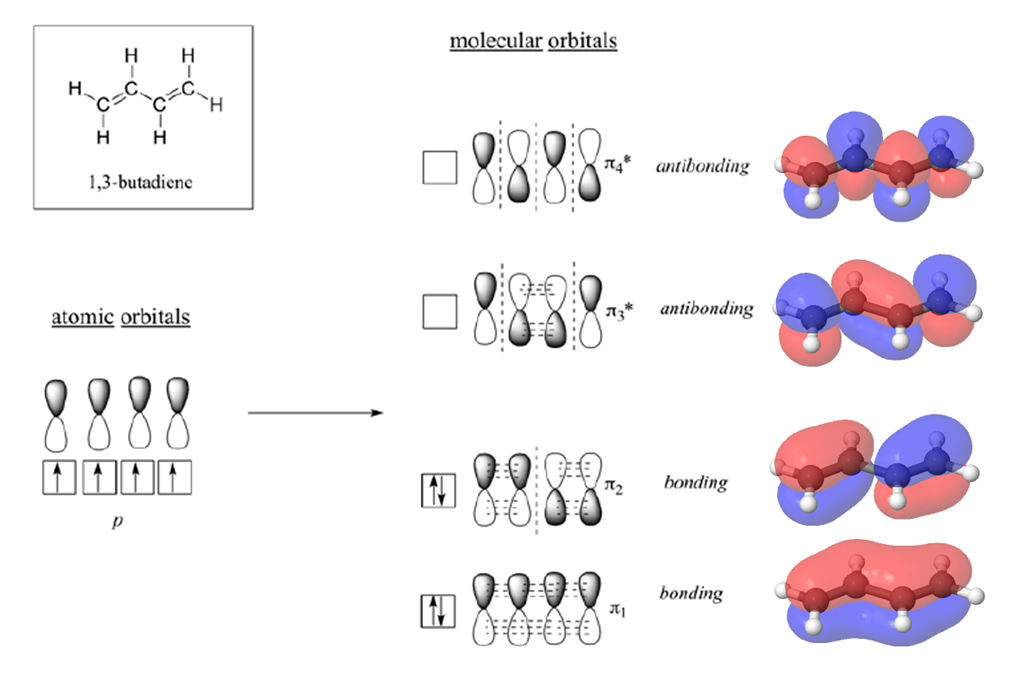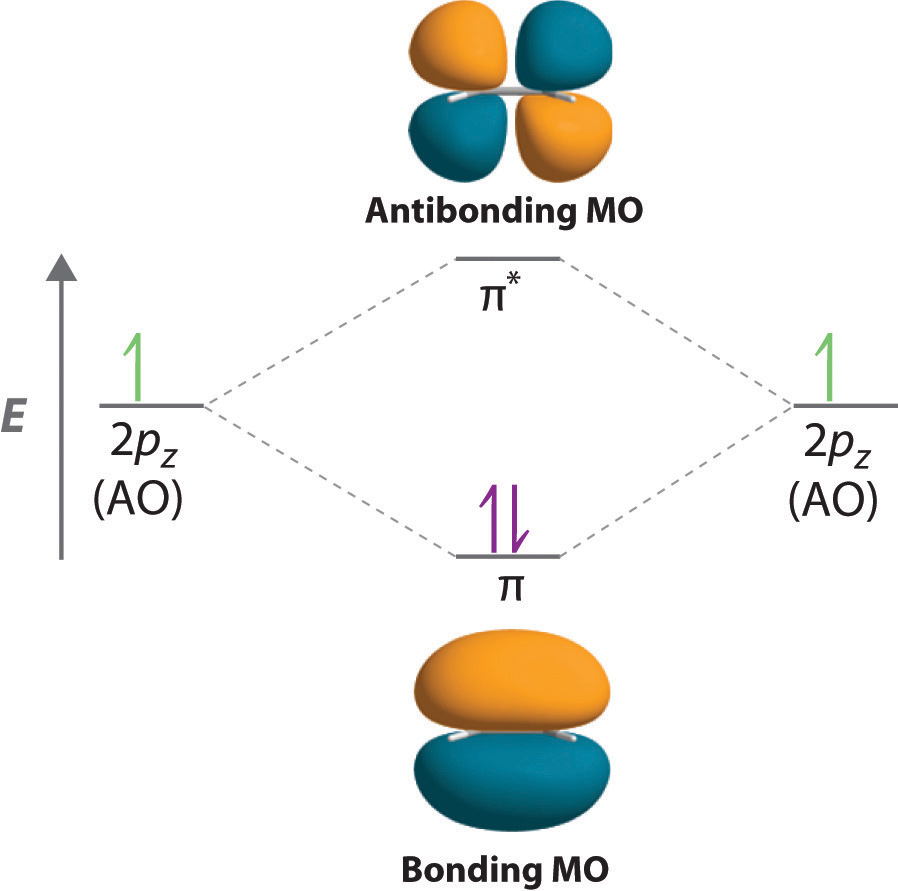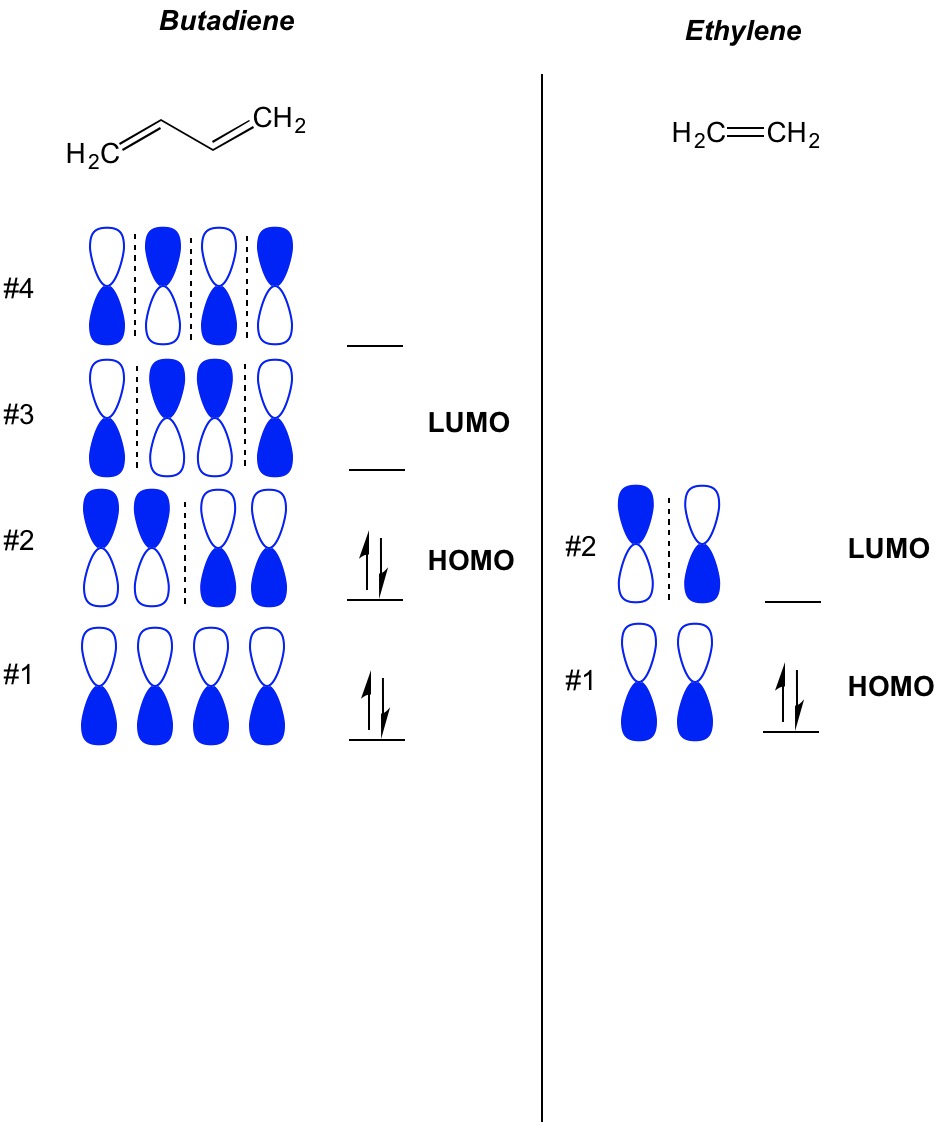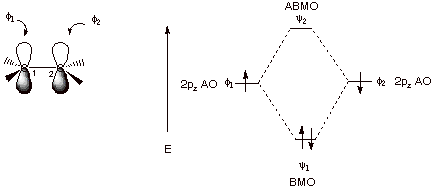
1. Strong Covalent Bonds. Consider the pi bond of ethene in simple molecular orbital terms (The qualitative results would be the same for any pi or sigma bond.

Rationalize the differences between the \pi orbitals of butadiene and acrolein (use MO diagram). | Homework.Study.com
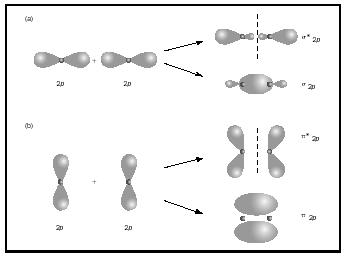
Molecular Orbital Theory - Chemistry Encyclopedia - structure, number, molecule, atom, Bond Order, Multiple Bonds

organic chemistry - Draw a simplified MO diagram for the pi system of Methyl vinyl ether - Chemistry Stack Exchange

organic chemistry - Draw a simplified MO diagram for the pi system of Methyl vinyl ether - Chemistry Stack Exchange

The relative energy levels of the five pi molecular orbitals of the cyclopentadienyl system are similar to those in benzene. That is, there is a single lowestenergy MO, above which the orbitals

organic chemistry - Draw a simplified MO diagram for the pi system of Methyl vinyl ether - Chemistry Stack Exchange