![Highly selective and efficient extraction of two Pb2+ ions with a p-tert-butylcalix[6]arene hexacarboxylic acid ligand: an allosteric effect in extraction - RSC Advances (RSC Publishing) Highly selective and efficient extraction of two Pb2+ ions with a p-tert-butylcalix[6]arene hexacarboxylic acid ligand: an allosteric effect in extraction - RSC Advances (RSC Publishing)](https://pubs.rsc.org/en/Content/Image/GA/C3RA44289F)
Highly selective and efficient extraction of two Pb2+ ions with a p-tert-butylcalix[6]arene hexacarboxylic acid ligand: an allosteric effect in extraction - RSC Advances (RSC Publishing)
Efficient and selective removal of Pb2+ from aqueous solution by using an O− functionalized metal–organic framework - Dalton Transactions (RSC Publishing)
![SOLVED: Select the expression for the solubility product constant for Pblz from the list below: [Pb2+1[ 21-]? Ksp [Pblz] C. Ksp [Pb2+][I-]2 B. Ksp [Pblz] [Pbz*Jn-]7 DS Ksp [Pb?+][2I-]2 none of the SOLVED: Select the expression for the solubility product constant for Pblz from the list below: [Pb2+1[ 21-]? Ksp [Pblz] C. Ksp [Pb2+][I-]2 B. Ksp [Pblz] [Pbz*Jn-]7 DS Ksp [Pb?+][2I-]2 none of the](https://cdn.numerade.com/ask_images/11ab1dc9f5b4452f802e2868fcfaf063.jpg)
SOLVED: Select the expression for the solubility product constant for Pblz from the list below: [Pb2+1[ 21-]? Ksp [Pblz] C. Ksp [Pb2+][I-]2 B. Ksp [Pblz] [Pbz*Jn-]7 DS Ksp [Pb?+][2I-]2 none of the

SOLVED: 12) Considering the following precipitation reaction: Pb(NO3)2(aq) + 2KI(aq) Pbl2(s) + 2KNO3aq) Which ion would NOT be present in the complete ionic equation? A) I- B) K+ D) Pb2+ C)NO3 E)
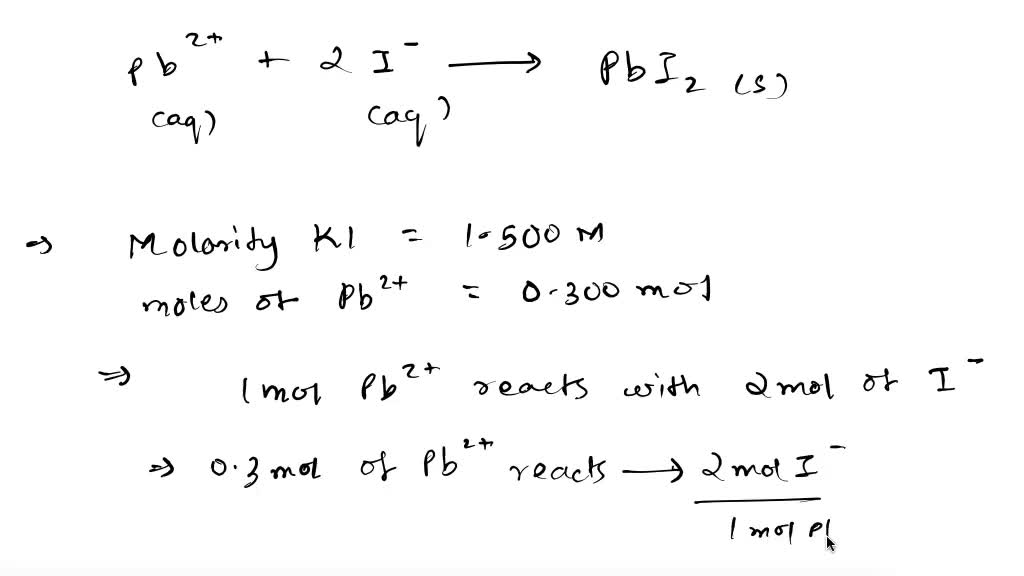
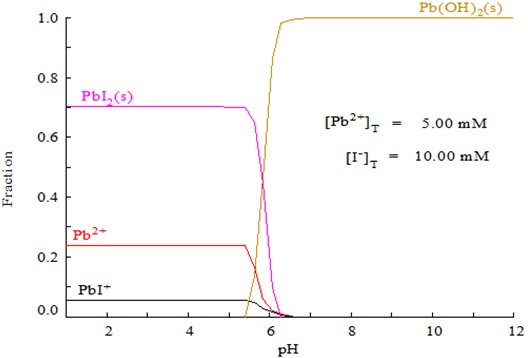
SOLVED: Lead ions can be precipitated from aqueous solutions by the addition of aqueous iodide: Pb2+(aq) + I-(aq) → PbI2(s) (Net ionic equation) Lead iodide is virtually insoluble in water so that
![SOLVED: What [Pb2+] should be maintained in Pb(NO3)(aq) to produce a solubility of 1.9×10−4 mol PbI2/L when PbI2(s) is added? Express your answer using two significant figures. The answers I found in SOLVED: What [Pb2+] should be maintained in Pb(NO3)(aq) to produce a solubility of 1.9×10−4 mol PbI2/L when PbI2(s) is added? Express your answer using two significant figures. The answers I found in](https://cdn.numerade.com/ask_previews/08fd4d3d-df52-4bbe-9540-0ab919346f25.gif)
SOLVED: What [Pb2+] should be maintained in Pb(NO3)(aq) to produce a solubility of 1.9×10−4 mol PbI2/L when PbI2(s) is added? Express your answer using two significant figures. The answers I found in

Pb2+ as a Substrate and a Cofactor of a Porphyrin Metalation DNAzyme - Yang - 2020 - ChemBioChem - Wiley Online Library

The removal of Pb2+ from aqueous solution using mangosteen peel activated carbon: Isotherm, kinetic, thermodynamic and binding energy calculation - ScienceDirect


![H2ampa─Versatile Chelator for [203Pb]Pb2+, [213Bi]Bi3+, and [225Ac]Ac3+ | Inorganic Chemistry H2ampa─Versatile Chelator for [203Pb]Pb2+, [213Bi]Bi3+, and [225Ac]Ac3+ | Inorganic Chemistry](https://pubs.acs.org/cms/10.1021/acs.inorgchem.2c00636/asset/images/acs.inorgchem.2c00636.social.jpeg_v03)