Class 12- D block element) Q.Why zinc is not considered as d block element or transition element? - YouTube

Why do the transition elements have higher enthalpies of atomization? In 3d series ( Sc to Zn ), which element has the lowest enthalpy of atomization and why?

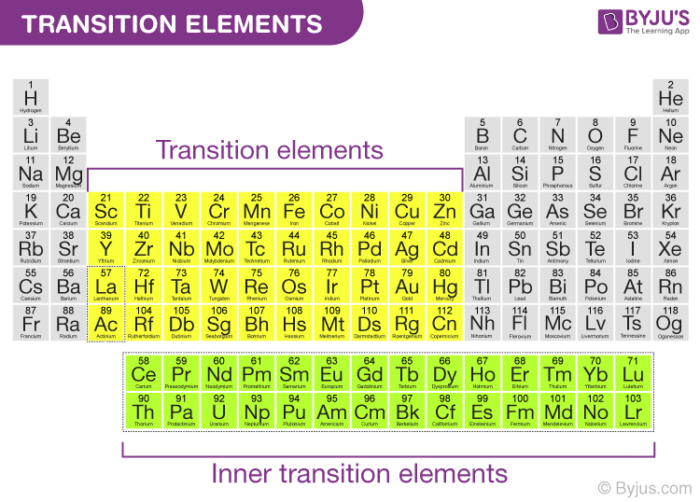
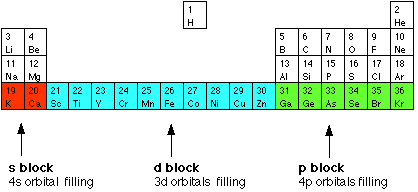
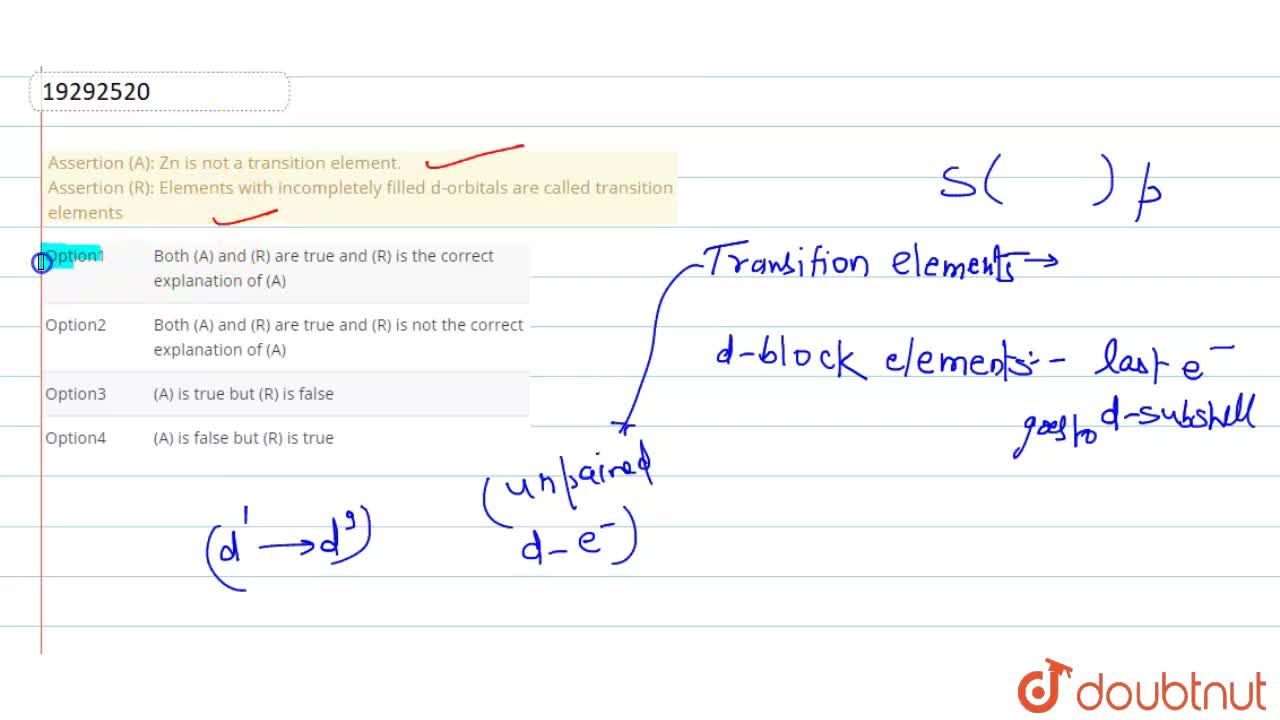
Given reasons : Zn is not regarded as a transition element. (ii) Cr^(2+) is a strong reducing agent.

On what ground can you say that scandium (Z = 21) is a transition element but zinc (Z = 30) is not? - YouTube
Why are scandium and zinc not regarded as transition metals despite their been in the transition series? - Quora

Chapter 15: Transition Metals 15.1 General Properties of Transition Metals 15.2 Complex Formation and the Shape of Complex Ions 15.3 Coloured Ions ppt download